Epigenic's Latest Research Published in Nature Biotechnology: Novel Epigenetic Regulator Enables Highly Efficient and Long-Term Cholesterol-Lowering in NHPs
October 1, 2025, Epigenic published a paper in the prestigious international journal Nature Biotechnology, titled "Design of optimized epigenetic regulators for durable gene silencing with application to PCSK9 in non-human primates". Through systematic optimization of epigenetic regulatory tools, this study developed a novel and highly efficient regulator, EpiReg-T. In non-human primates (NHPs), EpiReg-T achieved a significant therapeutic effect with a single administration: it stably inhibited PCSK9 gene expression and reduced "bad cholesterol" (low-density lipoprotein cholesterol, LDL-C) for a long time. This provides a potential new therapeutic strategy for cardiovascular and other metabolic diseases.

As an emerging direction in the field of gene therapy, epigenetic regulation does not alter DNA sequences. Instead, it precisely controls the "activation" or "silencing" of genes by regulating their epigenetic modifications, offering a new approach to enhancing the safety of gene therapy. However, the clinical translation of this technology has been hindered by the challenge of achieving long-lasting effects. The research team addressed this by systematically optimizing the components and architecture of the epigenetic regulators, leading to significantly enhanced efficiency. Direct comparison in cells, mice, and cynomolgus monkeys demonstrated that the single-component regulator based on TALE protein (EpiReg-T), which has a simpler structure and higher delivery efficiency, exhibited superior efficacy at lower doses compared to the two-component CRISPR/dCas9-based system. In the NHP model, a single intravenous administration of EpiReg-T resulted in sustained and profound suppression of blood PCSK9 and LDL-C levels. This therapeutic effect remained stable for over a year at the time of publication, with no signs of reversal. Furthermore, the study revealed that this durable silencing results from the synergistic action of DNA methylation and histone modification, deepening the understanding of the underlying molecular mechanisms of epigenetics.
To create an epigenetic regulator with optimal performance, the research team conducted a systematic screening of versions with different components and structures in mice. They delivered mRNA encoding the regulators to the liver via lipid nanoparticles (LNPs) and ultimately identified two optimal versions: the dCas9-based EpiReg-C and the TALE-based EpiReg-T. In head-to-head comparisons with previously reported zinc-finger protein-based epigenetic regulators and the base editor ABE8.8, both EpiReg-C and EpiReg-T demonstrated significant efficiency advantages (Fig. 1c). Further comparison across models revealed that while both achieved similar levels of suppression at saturating doses, EpiReg-T showed greater dose sensitivity, meaning it achieved potent effects at significantly lower doses, a characteristic also confirmed in NHPs (Fig. 1d).
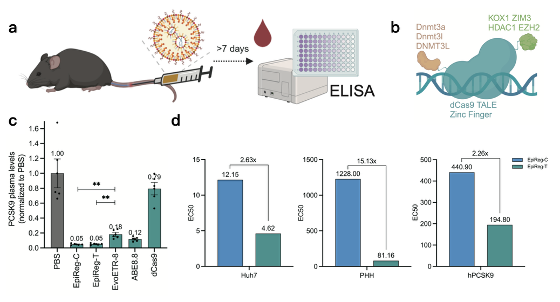
Fig. 1: The screening and comparison of EpiReg
To verify whether the gene silencing could be stably maintained through cell division ("inherited"), the team performed a two-thirds partial hepatectomy in mice (Fig. 2a). Results showed that PCSK9 suppression remained stable even after rapid liver regeneration (Fig. 2b). Additionally, this epigenetic suppression exhibited a crucial safety feature: reversibility. After achieving deep PCSK9 suppression, administration of an epigenetic activator tool restored expression levels to baseline (Fig. 2c).
In the critical NHP experiment, a single high dose (2.25 mg/kg) of EpiReg-T achieved approximately 90% PCSK9 suppression and about 60% reduction in LDL-C. Moreover, the therapeutic effect remained stable during a continuous follow-up period of 343 days (Fig. 2d, e).
At the molecular mechanism level, the study uncovered the secret to the long-lasting silence: EpiReg-T simultaneously established two repressive "marks" – DNA methylation and the histone modification H3K27me3 – at the target gene's regulatory region, while significantly reducing activating marks (H3K4me3, H3K9ac, H3K27ac) that promote gene tranion (Fig. 2f, g).
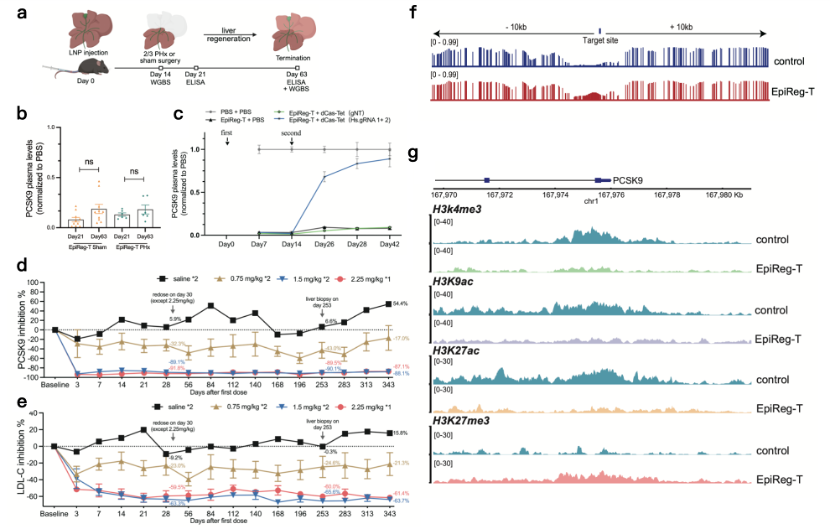
Fig. 2: Validation of EpiReg-T in mice and non-human primates
Regarding safety, whole-genome sequencing and analysis of potential off-target sites predicted by sequence homology confirmed that EpiReg-T did not cause off-target effects even at saturating doses, demonstrating high targeting precision.
This achievement marks a key progress in the advancement of epigenetic regulation technology toward clinical application, highlighting its significant advantages in safety, durability, and efficacy. In the future, Epigenic will continue to strengthen its innovative gene therapy platform, accelerate the clinical translation of breakthrough scientific discoveries, and bring new hope to patients worldwide.



